Pharma Commercialization Services
Your trusted partner in achieving commercial excellence
At Thermo Fisher Scientific, we recognize the complexities and challenges of bringing pharmaceutical products to market. Our comprehensive commercial manufacturing services provide pharmaceutical and biotechnology companies with a trusted partner to seamlessly navigate the journey from development to commercialization.
Our end-to-end solutions include drug substance (API) and drug product manufacturing, packaging, and distribution, offering a streamlined approach to meet your manufacturing needs. Utilizing our global network of state-of-the-art facilities and deep industry expertise, we deliver high-quality, regulatory-compliant manufacturing services tailored to your specific requirements.
With a focus on quality, efficiency, and innovation, we collaborate closely with our clients to accelerate timelines, mitigate risks, and optimize manufacturing processes. From technology transfer and scale-up to ongoing production and supply chain management, we are dedicated to supporting your success at every stage.
Explore our specific service offerings for tech transfers, commercial packaging at any scale, and commercial manufacturing principles.
Our commercial manufacturing services
Thermo Fisher Scientific offers reliable and simple technology transfer services to facilitate the seamless transfer of pharmaceutical manufacturing processes from development to commercial-scale production.
Key advantages of Thermo Fisher's tech transfer services include:
- Expertise and experience at a global scale
- Harmonized tech transfer process across a global network of sites
- Proactive risk management
- Use of digital modeling to streamline and shorten tech transfer timelines and mitigate potential risks
- Digital project management tools and customer platforms for increased visibility of project progress
- Regulatory support and quality assurance compliance on a global level
- Customized and flexible tech transfer solutions and collaborative approach

To learn more about our tech transfer services, contact a tech transfer specialist.
Maintaining a stable drug supply in commercial manufacturing is crucial for consistent production, quality, and availability of medications. Partnering with a global entity that has a well-established network of production sites is essential. Additionally, auxiliary services like total transportation management, a fully audited global depot network, import record management, and VAT reclaim capabilities ensure an uninterrupted drug supply, which is key to success.
Thermo Fisher collaborates closely with clients as their strategic and trusted partner, providing expert guidance, technical support, and project management throughout the manufacturing lifecycle to achieve shared goals and objectives.
Working with Thermo Fisher Scientific provides the following advantages:
- Ensured supply chain security and resilience
- Proactive and robust risk management processes
- Advanced quality and in-process quality control and assurance
- Integrated quality systems
- Regulatory support at a global level
- Flexibility at scale
- Digital project management platforms
- Ability to pack HiPo and LoPo products

Contact us to learn more about our commercial manufacturing capabilities.
Identifying the right packaging strategies is crucial for the successful commercialization of your product. Leverage our commercial facilities in the EU, United Kingdom, and United States, along with our extensive range of capabilities, to accommodate both specialty and primary care products. This ensures your product is packaged to the highest quality standards with cost-effective solutions, regardless of the volume required.
Our breadth and depth of commercial packaging capabilities are designed to accommodate both small and large volumes to ensure that your product can be packaged in the most cost-effective way possible.

Take advantage of Thermo Fisher Scientific’s packaging services to:
- Ensured supply chain security and resilience
- Proactive and robust risk management processes
- Simplify your logistics
- Deliver proven technical packaging knowledge and experience
- Provide reliable service levels and focus on quality
- Enable flexibility for both small- and large-volume projects
- Streamline timelines with end-to-end solutions from development to clinical to commercial
- Allow flexibility for changes in capacity for product growth or contingency planning
With an integrated pharma services network of sites offering a variety of upstream and downstream capabilities, our experienced project managers and cross-functional technical team will ensure your project's success. Whether your project is entering the clinical phase or nearing commercialization, our global network provides you with an end-to-end supply chain solution.
Flexible and broad packaging capabilities designed around your needs with a global network of sites:
- Oral solid dose packaging (bottle/blister/sachet)
- Sterile packaging (vial/ampoule/syringe/pre-filled syringe/autoinjector pen assembly)
- Kit assembly
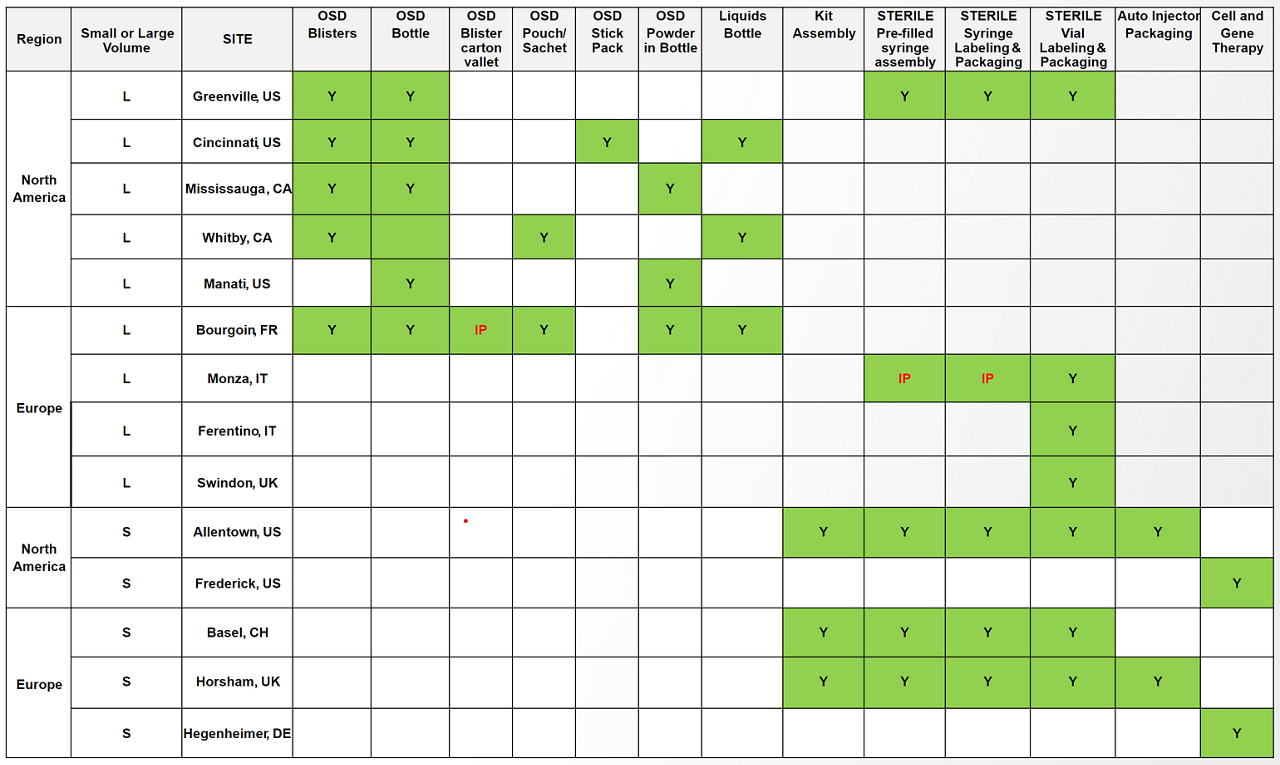
Site capabilitites
Contact us to get more information on our small or large commercial-scale packaging capabilities.
Our strong and diverse regulatory expertise, based on solid knowledge of the requirements of major entities (ICH/EMA/FDA) and a commitment to staying abreast of new regulatory trends, is the foundation that enables us to deliver exceptional services continuously recognized by our customers.
Visit our regulatory services and support page to learn more, or contact our regulatory specialists.
Our clinical trial packaging services ensure that your investigational products are packaged with precision and compliance, meeting the highest standards of quality and regulatory requirements. From labeling to distribution, we provide tailored solutions that support the unique needs of your clinical trials, ensuring seamless and efficient delivery to trial sites worldwide.
Thermo Fisher’s fully owned cGMP facilities support ambient, refrigerated, and frozen packaging capabilities, and are strategically located around the globe to accommodate regional needs. An integrated IT system links the facilities to give clients control over inventory via barcode standards. Our global clinical packaging services include primary packaging, secondary packaging, pre-filled syringe assembly and labeling, and smart packaging to improve medication adherence.
Labeling: Producing clinical trial labels demands greater oversight and management than commercial labeling to maintain the integrity of blind studies and comply with stringent regulatory controls. Our advanced inspection software, comprehensive in-house printing capabilities, translation and regulatory approval management, and centralized web-based document management platform are specifically designed to reduce overall label cycle times.
Visit our clinical trial label services page to learn more.
Total transporation management/distribution: Our logistics experts manage the storage and distribution of labeled and packaged ambient and cold chain clinical trial materials, investigational and comparator medicinal products, and placebos. We offer comprehensive import/export services, including Importer of Record (IOR) capabilities in over 24 countries, as well as handling returns and the destruction of supplies across our extensive network.